RaDaR Recruitment Documentation
Should a patient withdraw their consent, the RaDaR team must be notified as soon as possible. The RaDaR team will action the request to withdraw. Do not delete any information from RaDaR. This can have unexpected effects on the database.
For a step-by-step guide to recruitment to RaDaR please visit the Recruiting Patients page.
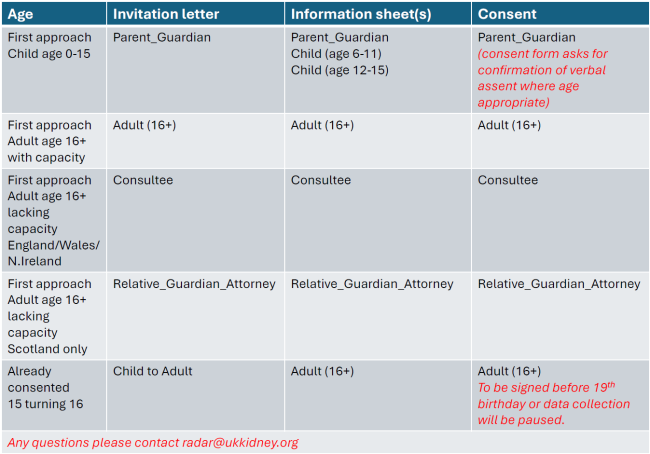
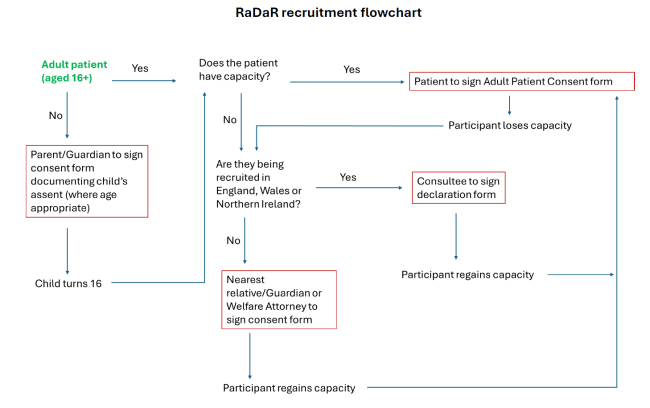
At the bottom of this page, we provide a table and flow chart to help you decide which documents should be issued to potential RaDaR participants. The table and flow chart are also in the protocol.
RaDaR Protocol
RaDaR Invitation Letters, Information Sheets and Consent Forms
Child (0-15)
- RaDaR 2024 Parent_Guardian Invitation Letter (0-15) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Parent_Guardian Information Sheet (0-15) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Child Patient Information Sheet (age 6-11) v2 19.12.2024 (PDF) (.docx)
- RaDaR 2024 Child Patient Information Sheet (age 12-15) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Parent/Guardian Consent Form (0-15) v2 05.08.2025 (PDF) (.docx)
Child to adult (15 turning 16) with capacity
- RaDaR 2024 Child to adult Invitation Letter (15 turning 16) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Adult Patient Information Sheet (16+) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Adult Consent Form (16+) v2 05.08.2025 (PDF) (.docx)
Adult (16+) with capacity
- RaDaR 2024 Adult Invitation Letter (16+) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Adult Patient Information Sheet (16+) v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Adult Consent Form (16+) v2 05.08.2025 (PDF) (.docx)
Adult (16+) without capacity - England, Wales and Northern Ireland
- RaDaR 2024 Consultee Invitation Letter v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Consultee Information Sheet v2 19.12.2014 (PDF) (.docx)
- RaDaR 2024 Consultee Declaration Form v3 05.08.2025 (PDF) (.docx)
Adult (16+) without capacity - Scotland
- RaDaR 2024 Relative_Guardian_Attorney Invitation Letter v1 06.09.24 (PDF) (.docx)
- RaDaR 2024 Relative_Guardian_Attorney Information Sheet v2 19.12.24 (PDF) (.docx)
- RaDaR 2024 Relative/Guardian/Attorney Consent Form v3 05.08.2025 (PDF) (.docx)
Table and flow chart